RevBio announced that it has received approval from the U.S. Food and Drug Administration to commence a 20-patient clinical trial to assess the safety and efficacy of a more rapidly replaced, pH-modified, porous formulation of the company’s bone adhesive biomaterial called Tetranite. The trial aims to immediately stabilize dental implants after tooth extractions. This new formulation has exhibited evidence of a more biologically active bone substitution. While not osteoinductive, this patent-pending version of Tetranite demonstrates characteristics that may be described as “osteopromotive.”
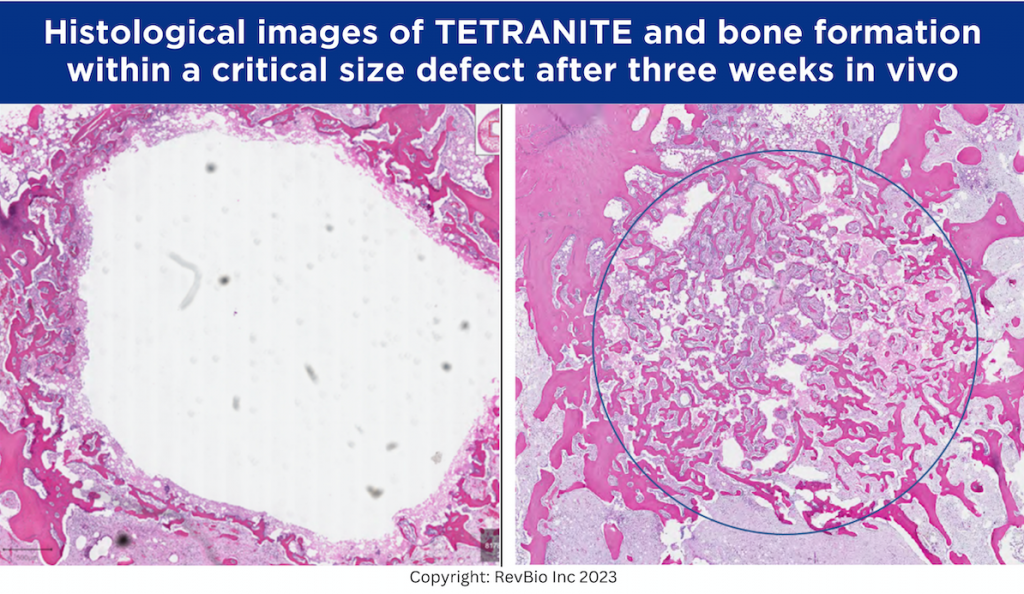
Above are histological images of bone formation within a critical size defect after three weeks in vivo in a rabbit distal femur. On the left, the original Tetranite formulation shows very little substitution with new bone and appears white, which indicates the presence of the biomaterial. On the right, the more osteopromotive formulation shows significant bone in-growth with the red and pink areas indicating new bone substituting the Tetranite biomaterial. (Photo: Business Wire)
The clinical trial will be conducted by Paul A. Fugazzotto, DDS, a world-renowned periodontist based in Massachusetts, with over 30 years of experience in dental implant placements, and Kanyon Keeney, DDS, an oral and maxillofacial surgeon based in Virginia, whose clinical practice exclusively focuses on dental implant surgery.
“This product is truly transformational,” said Dr. Fugazzotto. “Tetranite will revolutionize how implant dentistry will be performed. The adhesive properties and handling characteristics of this material are incomparable to any product on the market.”
Teeth are extracted due to damage from traumatic injuries, tooth decay, or gum disease. The current standard of care involves multiple staged surgical procedures to restore a patient’s dentition with prosthetic crowns supported by dental implants. However, extraction sites often prove too large for dental implants to achieve primary stability through conventional mechanical engagement.
As a consequence, patients must undergo a costly, complex, and lengthy process, including a preliminary bone grafting surgery before receiving a dental implant. Based on company surveys, each year in the U.S., approximately 2.1 million implants are eventually placed into initially unstable tooth extraction sites that first require a bone graft.
The use of Tetranite to stabilize an unstable implant will allow for the immediate placement of dental implants that could otherwise not be inserted until the initial bone graft has healed to form new bone.
As a result, the Tetranite biomaterial will help reduce the duration and complexity of these dental implant procedures, lessen patient pain and recovery time, and decrease the overall cost of care, thereby providing greater patient access for the treatment of tooth loss.
“We are truly excited to conduct this study with both Dr. Fugazzotto, who will initiate this study enrolling the first cases, along with Dr. Keeney,” said Alan Pollack, RevBio’s senior director of dental clinical operations. “The improvements we have made to the technology have accelerated both the bone substitution profile and the product’s adhesive strength. Having also just recently received IRB approval, we look forward to enrolling our first patients in the coming weeks.”
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company engaged in the development and commercialization of a patented, synthetic, injectable, self-setting, and osteoconductive bone adhesive biomaterial called Tetranite.
The company is initially developing this technology for use in the dental, cranial, and broader orthopaedic markets as well as applications in the animal health market.
RevBio’s Tetranite technology is not yet approved for commercial use.












