FDA Provides EUA for COVID-19 Treatment
Dimensions of Dental Hygiene
NOVEMBER 10, 2020
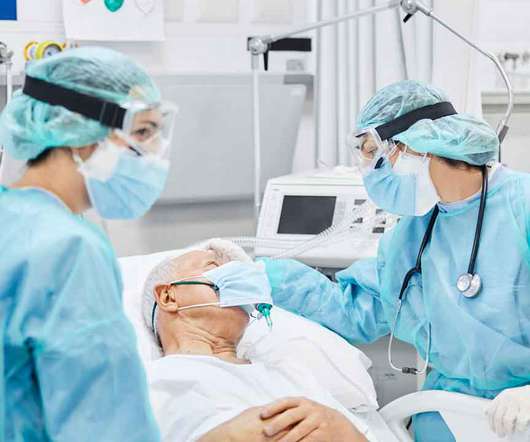
On November 9, the United States Food and Drug Administration issued an emergency use authorization (EUA) to bamlanivimab for the treatment of mild-to-moderate COVID-19. The post FDA Provides EUA for COVID-19 Treatment appeared first on Dimensions of Dental Hygiene.





Let's personalize your content